- Phone: 800-492-1252
- Fax: 440-368-3569
- E-mail: info@spartanwatertreatment.com
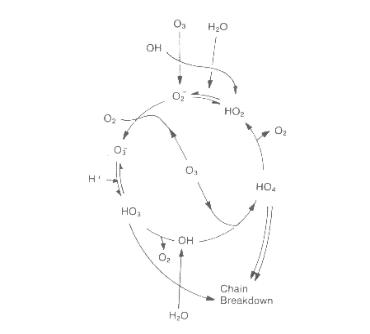
The stability of dissolved ozone is readily affected by pH, ultraviolet light, ozone concentration, and the concentration of radical scavengers. The decomposition rate, (aliphatic and aromatic) measured in the presence of excess radical scavengers, which prevent secondary reactions, is expressed by a pseudo first-order kinetics. Ozone decomposition occurs in a chain process that can be represented by reactions shown in Figure 1 below (based on the two most important models of Staehelin et al,Tomiyasu et al), including an initiation step, propagation steps, and break in chain reaction steps.
Initiators, Promoters and Inhibitors of free-radical reactions.
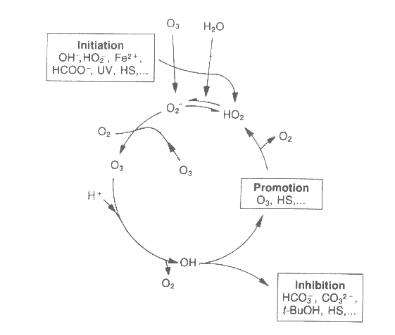
There are a wide variety of compounds able to initiate, promote, or inhibit the chain-reaction processes these are discussed below. Figure 2 shows where these compounds act in the ozone chain reaction.
The initiators of the free-radical reaction are those compounds capable of inducing the formation of superoxide ion O2
– from an ozone molecule. Those are inorganic compounds (hydroxyl ions OH-, hydroperoxide ions HO2 – and some cations), organic compounds (glyoxylic acid, formic acid, humic substances,…) and UV radiation at 253.7 nm.
Promoters of the free-radical reaction are all organic and inorganic molecules capable of regenerating the O2
– superoxide (which can promote the decomposition of ozone) anion from the hydroxyl radical. Common promoters that are also organics include aryl groups, formic acid, glyoxylic acid, primary alcohols and humic acids. Among the inorganic compounds, phosphate species are worth special mention.
The inhibitors of the free-radical reaction are compounds capable of consuming OH radicals without regenerating the superoxide anion O2-. Some of the more common inhibitors include bicarbonate and carbonate ions, alkyl groups, and tertiary alcohols (e.g. t-butanol).