- Phone: 800-492-1252
- Fax: 440-368-3569
- E-mail: info@spartanwatertreatment.com
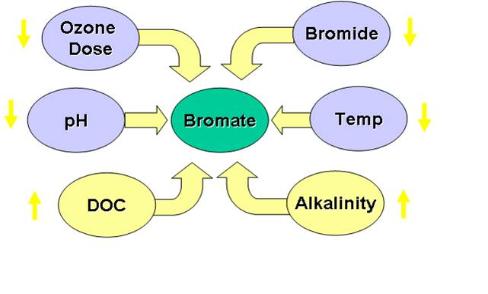
Bromate formation during ozonation may occur if sufficient levels of bromide are present in water during ozonation. The U.S. Environmental Protection Agency (EPA) has a limit of 10 ppb of bromate in drinking water. This limit exists because there is an increased risk of cancer from long-term exposure above the maximum contaminant level (MCL). Many drinking water sources contain bromide so the application of ozone must be done in a way that minimizes the potential to form this compound. The formation of bromate is a complicated process that involves both ozone and its decomposition product the hydroxyl radical.
The bromate formation diagram shows some of the factors that affect bromate formation during ozone drinking water treatment. The yellow arrows indicate how a change in a given factor will influence bromate formation. For example, decreasing the ozone dose will result in lower formation of bromate. So lowering ozone dose, pH, bromide ion concentration, and temperature will reduce bromate formation. Increasing levels of alkalinity and dissolved organic carbon will lower bromate levels. A more detailed model is shown below by Dr. Urs von Gunten at The Swiss Federal Institute of Aquatic Science and Technology (EWAG) in Zurich:

Understanding these factors and adjusting the ozone water treatment process properly can control the bromate level for most water treatment facilities below the EPA limits.
In general, producers of municipal drinking water or bottled water are always balancing the formation of disinfection byproducts such as bromate or trihalomethanes versus the control of pathogens in the water. The more difficult to kill the pathogen, the more disinfectant and time required. This combination of disinfection dose and time is referred to as the CT. It is the product of multiplying the in mg/l times the time usually recorded in minutes.

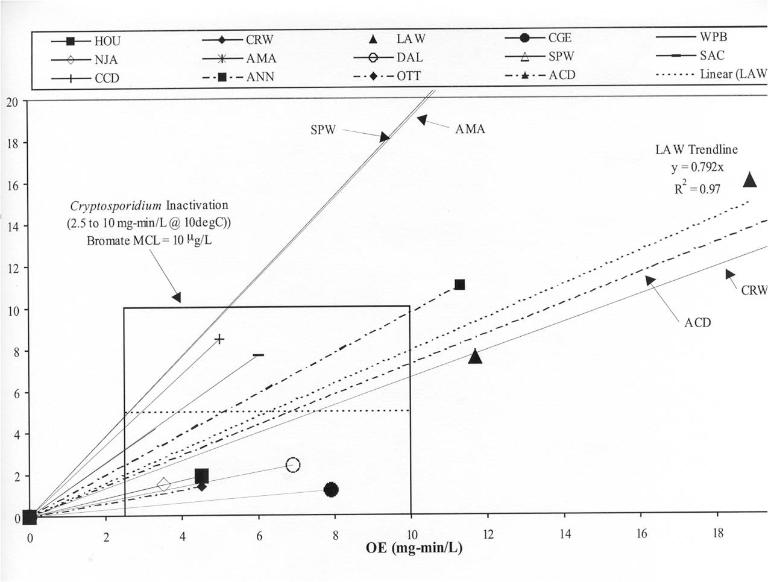
One of the most difficult to kill pathogens is Cryptosporidium. Thus the highest doses of ozone are used in trying to control this pathogen in water treatment. If simultaneous control of Cryptosporidium can be achieved with bromate control, most other disinfection applications would be expected to be safe as well.
The chart below shows a number of large drinking water plants with both bromate formation potential and CT for Cryptosporidium. The data indicate that most facilities with bromide in the water can meet the most stringent disinfection requirement while still staying below the EPA limit for bromate.
Contact Spartan Environmental Technologies if you have questions about bromate formation during ozonation.