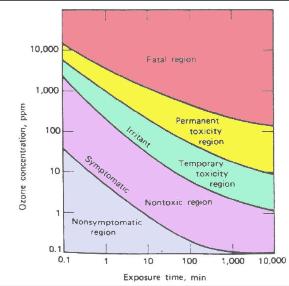
It is worthwhile to mention the toxic character of ozone, specially at high concentrations. While ozone is considered to be a toxic gas, there are factors which mitigate the immediate danger to individuals working with it. Toxicity is dependent on concentration and length of exposure. An exposure of less than 0.2 mg.m-3 can be tolerated indefinitely, 2 mg.m-3 (1 ppm) can be tolerated for 8 min, and up to 8 mg.m-3 (4 ppm) can be tolerated for one minute without producing the symptoms of coughing, eye watering, and irritation of the nasal passages. Learn more about ozone limits, precautions, exposure and view the ozone material safety data sheet (MSDS) below.
The U.S. OSHA ozone limits are 0.10 ppm for 40 hour exposure. The OSHA 15 minute limit is 0.30 ppm. The American Conference of Governmental Industrial Hygienists (ACGIH®) has set a TLV as ceiling of 0.2 mg.m-3 (0.1 ppm) for ozone. Equivalent parameters, called VLA (Valor Límite Ambiental), have been established in Spain, depending on the type of work. The VLA-ED values (equivalent to TLV-TWA) are: 0.05 ppm (0.1 mg.m-3) for heavy work, 0.08 ppm (0.16 mg.m-3) for moderate work, 0.1 ppm (0.2 mg.m-3) for light work and 0.2 ppm (0.4 mg.m-3) for times of exposure lower than 2 hours
There are other factors which lessen the risk to personnel working with ozone. The odor threshold concentration for ozone is about 0.02-0.04 mg.m-3 (0.01-0.02 ppm). Thus, ozone is generally detected by personnel before dangerous concentrations are reached. Moreover, once a critical concentration is reached, the results are not immediately toxic but merely symptomatic.
The following link provides a chart on ozone toxicity as a function of concentration and time in PDF format.
The following MSDS describes the hazards and the precautions for ozone:
Product: Ozone
1. Product Identification
Synonyms: Triatomic oxygen
CAS No.: 10028-15-6
Molecular Weight: 48.0
Chemical Formula: O3
2. Composition/Information on Ingredients
| Ingredient | CAS No | Percent | Hazardous |
| Ozone gas | 10028-15-6 | 1 – 15% | Yes |
3. Hazards Identification
Emergency Overview of Ozone
————————————————-
Highly reactive, can explode on contact with organic substances, especially strong reducing agents.
Ozone is a powerful oxidizing agent and oxidation with ozone evolves more heat and usually starts at a lower temperature than oxidation with oxygen. It reacts with non-saturated organic compounds to produce ozonides which are unstable and may decompose with explosive violence. Ozone is an unstable gas which, at normal temperatures, decomposes to diatomic oxygen. At elevated temperatures and in the presence of certain catalysts such as hydrogen, iron, copper and chromium, this decomposition may be explosive.
Potential Health Effects of Ozone
————————————————–
Ozone Inhalation: Ozone causes dryness of the mouth, coughing, and irritation the nose, throat, and chest. It may cause labored breathing, headache, and fatigue. However, the characteristic sharp, irritating odor is readily detectable at low concentrations (0.01 to 0.05 ppm).
Skin: Absorption through intact skin is not expected.
Eye Contact: Ozone is an irritant to the eyes causing pain, lacrimation, and general inflammation.
Ingestion: It is not a route of exposure.
Aggravation of Pre-existing Conditions: Ozone may increase sensitivity to bronchoconstrictors including allergens.
4. First Aid Measures
Ozone Inhalation: Remove to fresh air. If breathing is difficult a trained person should administer oxygen. If respiration stops, give mouth- to-mouth resuscitation. Get professional medical attention.
Ingestion: Not an expected route of exposure.
Skin Contact: Wash skin thoroughly with soap and water.
Eye Contact: Immediately flush eyes with large amounts of water for at least 15 minutes while forcibly holding eyelids apart to ensure flushing of the entire eye surface. If irritation, pain, or other symptoms persist seek professional medical attention.
Acute Ozone Exposure: May cause irritation of skin, eyes, and mucous membranes of the respiratory tract. Drowsiness, dizziness, headache, and fatigue have been associated with exposure.
Chronic Ozone Exposure: Long-term health effects are not expected from exposures to ozone. A partial tolerance appears to develop with repeated exposures.
5. Fire Fighting Measures
Flash Point: N/D
Auto ignition Temperature: N/D
Flammable Limits in air, % by volume – Upper: N/D Lower: N/D
Extinguishing Media: Use extinguishing media suitable for surrounding fires.
Unusual Fire and Explosion Hazard: None expected, since ozone is highly unstable and decomposes under all conditions and is not encountered except at very small levels in the immediate vicinity where formed.
6. Accidental Release Measures
Evacuate danger area. Open doors and windows to allow area to ventilate. Consult an expert.
Ozone should be contained within a chemically compatible piping system.
Ozone is a powerful oxidizing agent and oxidation with ozone evolves more heat and usually starts at a lower temperature than oxidation with oxygen. It reacts with non-saturated organic compounds to produce ozonides which are unstable and may decompose with explosive violence. Ozone is an unstable gas which, at normal temperatures, decomposes to diatomic oxygen.
7. Handling and Storage
Handle in accordance with good industrial hygiene and safety practice. Ozone is to be contained within controlled and enclosed areas and transported from generation point to application point with ozone resistant hose or pipe.
8. Exposure Controls/Personal Protection
Exposure Guidelines: OSHA PEL: 0.10-ppm PEL/TLV
Ventilation Requirements: General exhaust recommended. Avoid working with ozone generating equipment in enclosed spaces.
Specific Personal Protective Equipment
Respiratory: Respirators may be used when engineering and work practice controls are not technically feasible, when such controls are in the process of being installed, or when they fail and need to be supplemented. Respirators may also be used for operations which require entry into tanks or closed vessels, and in emergency situations.
Only appropriate respirators shall be provided and used when the use of respirators is the only means of controlling exposure for routine operations or during an emergency. (Refer to Table 1 of ANSUI/ASTM E591-77 for appropriate respirator selection ).
Positive pressure air line with mask or self-contained breathing apparatus should be available for emergency use.
Goggles: Not necessary
Gloves: Not necessary.
Other Clothing and Equipment: Not necessary.
9. Physical and Chemical Properties
Specific Gravity (H2O=1): 2.144 g/L
Molecular Weight: 48.00
Boiling Point: -111.°C
Melting Point: -192.°C
Vapor Pressure: N/A
Evaporation Rate (BuAc=1): N/A
Vapor Density (Air=1): 1.7
Solubility in H2O % by Weight: 0.49
Appearance and Odor: Colorless to bluish gas with a characteristic pungent odor similar to the smell after strong lightning storms.
10. Stability and Reactivity
Stability: Ozone spontaneously decomposes under ordinary conditions so that it is not encountered except in the immediate vicinity of where it was formed. The rate of decomposition is increased by solid surfaces and by many chemical substances.
Hazardous Decomposition Products: Free radical oxygen.
Hazardous Polymerization: Will not occur.
Incompatibilities: Ozone is a powerful oxidizing agent and reacts with all oxidizable materials, both organic and inorganic. Some reactions are highly explosive: Alkenes, benzene and other aromatic compounds, rubber, dicyanogen, bromine diethyl ether, dinitrogen tetroxide, nitrogent trichloride, hydrogen bromide, and tetrafluorohydrazine.
11. Toxicological Information
Ozone is extremely irritating to the upper and lower respiratory tract. The characteristic odor is readily detectable at low concentrations (0.02 ppm to 0.05 ppm). Ozone produces local irritation of the eyes and mucous membranes and may cause pulmonary edema at high exposure. Systematically, ozone has been reported to mimic the effects of ionizing radiation, and may cause damage to chromosomal structures. A partial tolerance appears to develop with repeated exposures. Although most effects are acute, the possibility of chronic lung impairment should be considered based upon animal experimentation.
12. Ecological Information
Environmental Fate: No information found.
Environmental Toxicity: No information found.
13. Disposal Considerations
Do not dispose of ozone off gas to atmosphere without properly designed off gas destruct unit. State and local disposal regulations may differ from federal disposal regulations.
14. Transport Information
Proper Shipping Name: N/A
Hazard Class: N/A
Identification Number: N/A
Packing Group: N/A
15. Regulatory Information
SARA TITLE III: N/A
TSCA: The ingredients of this product are on the TSCA Inventory List.
OSHA: Nonhazardous according to definitions of health hazard and physical hazard provided in the Hazard Communication
Standard (29 CFR 1910.1200)
16. Other Information
Label Hazard Warning: HIGHLY REACTIVE. OZONE GAS AFFECTS THE RESPIRATORY SYSTEM.
Label Precautions: Keep away from heat, sparks and flame. Avoid contact with eyes, skin and clothing. Avoid breathing. Use with adequate ventilation.
Label First Aid: If inhaled, remove to fresh air. Get medical attention for any breathing difficulty.
Product Use: Laboratory Reagent, Water Treatment Chemical
Revision Information: Pure. New 16 section MSDS format, all sections have been revised.
Spartan Environmental Technologies L.L.C. (Spartan) provides the information contained herein in good faith but makes no representation as to its comprehensiveness or accuracy. This document is intended only as a guide to the appropriate precautionary handling of the material by a properly trained person using this product. Individuals receiving the information must exercise their independent judgment in determining its appropriateness for a particular purpose. SPARTAN MAKES NO REPRESENTATIONS OR WARRANTIES, EITHER EXPRESS OR IMPLIED, INCLUDING WITHOUT LIMITATION ANY WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE WITH RESPECT TO THE INFORMATION SET FORTH HEREIN OR THE PRODUCT TO WHICH THE INFORMATION REFERS. ACCORDINGLY, SPARTAN WILL NOT BE RESPONSIBLE FOR DAMAGES RESULTING FROM USE OF OR RELIANCE UPON THIS INFORMATION.