Introduction
Sewage and industrial plants located near residential areas can be subject to political and legal problems if these facilities produce unpleasant odors. In the case of a sewage plant and the associated collection system, chemicals or enzymes may be added to the liquid phase to prevent the formation of the odors. Mechanical changes to the sewage pump stations may also be made to reduce the odor. When these methods are not sufficient to control the odor, some form of odor control in the vapor phase may need to be applied.
This paper discusses the nature of sewage plant odor and vapor phase treatment options. It will focus on design consideration for chemical scrubbing systems especially those that employ ozone as an oxidant. Click here to down load a PDF of this article on Municipal Odor Control .
Causes of Odors
The substances responsible for the diffusion of odors into the atmosphere in the vicinity of treatment plants are generally gaseous inorganic products or highly volatile organic compounds. The former are mainly the result of biological activity in the sewage, the latter are often caused by the presence in the sewer of industrial wastes. The following compounds are associated with bad odors: mercaptans, skatoles, indoles, inorganic acids, aldehydes, ketones and organic compounds containing nitrogen or sulfur atoms. These compounds can originate from the anaerobic decomposition of compounds with a high molecular weight, especially proteins. These are recognized as being among the causes of bad- smelling odors at the outlet of sewer lines and in treatment plants in general.
Among the inorganic compounds, ammonia and hydrogen sulfide are considered to be the main causes of odor when the sewage comes from mainly households. The presence of hydrogen sulfide is caused by a reducing environment, i.e. characterized by low values of the oxidation-reduction potential. In fact, at estimated potentials ranging between 150-350 mV conditions are favorable to develop sulfur-reducing micro-organisms.
In gravity sewer systems, in which the speed of the sewage flow allows for continuous re-aeration, there is no significant production of hydrogen sulfide and other odor causing by-products. Under such circumstances, however, it has been determined that odors can still find the favorable conditions to form in the sludge film that covers the submerged surface of the sewer lines. The hydrogen sulfide that is formed in these areas, diffusing into the overlying sewage, lowers the Redox potential, counter-balancing the opposite effect of the natural re-aeration. It can therefore be understood how septic conditions may arise in low-speed sewage which trigger very intense activities of the sulfate-reducing bacteria. These conditions can become more severe if the collection lines are particularly long and the system is located in a region that experiences high temperatures.
Measurement of Odors
The following are some parameters to express the concentration of odors:
Perceptibility Threshold (ATC: Absolute Threshold Concentration), defined as the minimum concentration that can be detected by 100% (in some cases by 50%) of the persons involved with an olfactory analysis. In some cases the geometric mean of the measurements of the single members is used.
Odor Number (TON: Threshold Odor Number), or the number of dilutions needed to reduce the concentration of the sample to the ATC.
Maximum Exposure Concentration (TLV: Threshold Limit Value). This represents the maximum concentration at which persons can be exposed for a period of 8 hours a day, 5 days a week and 50 weeks a year (weighted average over 8 hours), for a work life of 40 years.
Maximum Allowable Concentration (MAC: Maximum Allowable Concentration): Maximum concentration which should never be exceeded.
TABLE 1 reports the values of these indices relative to a series of compounds found in the atmosphere of sewage treatment plants.
| Compound | ATC (ppm) | TLV (ppm) | MAC (ppm) | Olfactory Sensation |
|---|---|---|---|---|
| Hydrogen Sulfide | 0.00047 | 10 | 50 (USA) 20 (UK) | Rotten Eggs |
| Ammonia | 46.8 | 25 | 37.5 (UK) | Pungent |
| Methyl Mercaptan | 0.0021 | 10 | Rotting Cabbage | |
| Carbon Dusulfide | 0.21 | Sweet/Pungent | ||
| Biphenyl Sulfide | 0.0047 | Burned Rubber | ||
| Dimethyl Sulfide | 0.001 | Rotting Vegetables |
Odor Control Technologies
If vapor phase control of odors is indicated, there are several choices:
Chemical Oxidation
Thermal Oxidation
Biological Treatment
Thermal Oxidation
Thermal oxidation systems essential burn odor causing compound either directly or catalytically with or without heat recapture. Typically they are used to deal with volatile organic compounds with odor control being a secondary benefit. Thermal oxidation treatment, though efficacious in some applications and compact, involves high installation and operating costs (using fuels as “oxidizing” material) which are recommended only in specific cases. As a result they are used only for very high strength odors or very difficult to treat compounds.
Bio Filters
Bio filters remove odor by capturing the odor causing compounds in a media bed where they are oxidized by naturally occurring micro organisms. These systems have a low profile and thus don’t obstruct views. They are also relatively simply to use and are effective if properly designed and maintain.
A major limitation of bio filters is the large land area required for installations. The size of the bio filter surface area is directly related to the airflow to be treated and the need to provide about a 45 to 60 second detention time. Poor bio filter performance is usually attributed to lack of moisture in the filter media. Other performance inhibitors are short circuiting, pH depression, and high temperatures. A concentration of ammonia greater than 35 ppm in the foul air stream may cause a toxic accumulation of ammonium in the media, leading to reduced ammonia removal efficiency. The need to keep the bio filters moist results in a significant amount of water usage and the need to treat or dispose of leachate and condensate. Design criteria are not well established and bio filters may not be appropriate for very strong odors.
Chemical Scrubbing and Oxidation
Multi stage scrubbers can remove a wide range of odor causing compounds both acidic and basic. They have been proven to be effective in many applications. Typically these systems are employed with high intensity odors in large air volumes. There are several types of wet scrubbers including packed bed, mist, and venturi scrubbers. All are designed to maximize the contact between the odorous compounds of the foul air stream and a “scrubbing” chemical solution. The compounds are absorbed and then oxidized by the chemicals.
Packed beds use a shower of scrubbing liquid over a bed of high-surface-area plastic media to promote droplet and film contact within a reaction chamber. The foul air is ventilated through the plastic media in a direction that is co-current or counter-current to the liquid flow. The advantage of a packed scrubber is that the concentration of the scrubbing solution can be varied in response to fluctuating odor levels. These units are usually the least costly method of treating high intensity odors.
The size of chemical scrubbing systems is intermediate between thermal and biological systems as are the operating costs. A disadvantage of wet chemical scrubbing systems using hypochlorite is a potential for emission of chlorinated compounds and particulate from the scrubber exhaust stack, as well as a potential for emission of a bleach odor if chemical feed is not properly controlled. The use of ozone as the oxidant can minimize these problems.
Wet chemical scrubbing using ozone are a good solution for odor control in situations where there is high intensity odor, high air volumes, or limited space to site an odor control system. The use of ozone prevents the formation of chlorinated by products and because ozone is generated on site, it eliminates the need to purchase and store chlorine or hypochlorite. Ozone is also a more powerful oxidant than hypochlorite.
Design Considerations for Ozone Based Odor Control Systems
Some significant factors in designing odor control systems are: type of odor, odor concentration, temperature, specific ozone dose, contact chamber retention time, waste water acceptability criteria and type of scrubbing system.
Pilot testing is often a good way to determine the efficiency of the proposed system and even more so when industrial wastes are involved. If the designers have extensive experience in odor control, it may be possible to design the system without pilot tests.
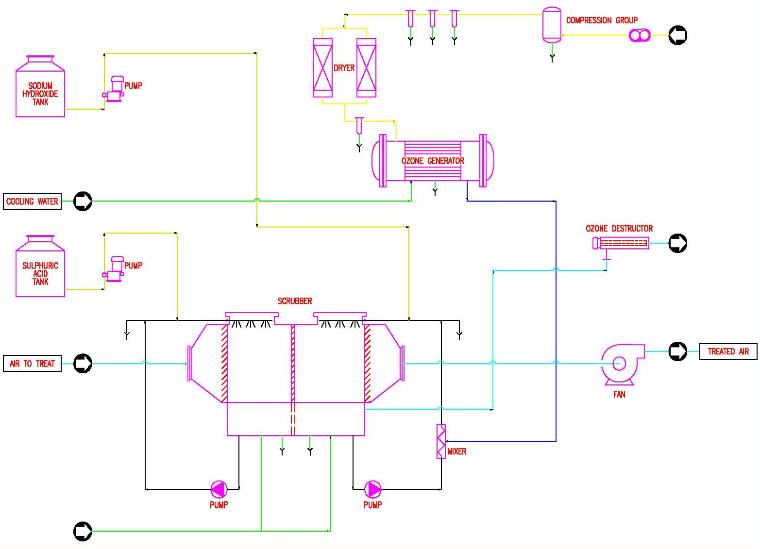
The air-liquid contact system is particularly important since the micro-pollutant must be transferred from the gaseous phase to the liquid phase to be oxidized by the ozone. As noted above there are several types of wet chemical scrubbing: backed bed, spray or venture. Consideration also has to be given to how to introduce the ozone into liquid phase prior to introduction into the scrubber. Ozone can be mixed with the liquid phase utilizing static mixers, ejectors or diffusers.
Recommended Odor Control Design
In treating air with both acidic and basic odor compounds, the scrubbing process includes two treatment phases, the first with acid (sulfuric acid) and the second with an alkaline oxidizer (caustic soda and ozone). This ensures the efficient removal of alkaline or acid organic and inorganic odor causing substances, viruses and bacteria. If only acidic odor compounds are present a single stage scrubber can be used.
Horizontally oriented back flushing type scrubber equipped with spray nozzles and a packed bed is recommended. The scrubbing liquids are recycled through the packed bed using centrifugal pumps. Addition of acid and base are controlled using pH monitors. Water addition is controlled by monitoring the levels in storage basins under the packed beds. Each scrubbing stage should be followed by a mist eliminator that can separate at least 90% of the particles with a diameter greater than 5 microns.
The system would monitor stack releases of oxidizer, ammonia and hydrogen sulfide use the results to control system. ORP measurement of the basic solution controls the flow of the amount of ozone introduced into the basic scrubbing solution. The ozone is introduced into the basic solution recycle line via a static in line mixer under the pressure from the ozone generator.
Scrubbing system and reagent used: In the first acid adsorption stage, typical consumption of acid to treat for basic compound such as ammonia is 40-60 gm of sulfuric acid (100% basis) /1000 N m3/hr of treated air. In the second stage where caustic soda is used in combination with ozone as the oxidizer, the typical consumption of caustic soda is 40-60 gm caustic soda (100% basis)/ 1000 N m3/hr of treated air. The cases considered typically had less than 10 ppm of hydrogen sulfide. The tanks for the acid and base should be sized for 15 days of storage.
Flow rates of exhausted air vary with unit operation:
The air is taken from the areas being deodorized by means of a duct system through a series of regularly spaced intakes that are connected to the piping by means of control dampers. Gas from any covered areas will be removed through openings located on the roof. Outdoor air for ventilation will be introduced through grilles made from anti-corrosive material which can be adjusted manually.
The piping coming from the single retention points will run into a single header that will transport the effluents to the scrubbing units. Flow rate control dampers will be inserted, where necessary, on the pipelines. The final header of the suction network will be connected to the scrubber. A control damper will be installed on the fan suction inlet.
Summary
Sewage and wastewater odors are most often associated with hydrogen sulfide and ammonia. These odors can be effectively treated in the vapor phase with thermal, biological and chemical treatment methods. The selection of which method works best depends on the concentration of the odor causing compounds, the air flow rate, available land area for the system, capital budget and discharge limitations for wastewater from the system. Chemical scrubbing using ozone as the oxidant is an effective choice for high intensity odors and high air volumes where the amount of space for the treatment system is limited.