- Phone: 800-492-1252
- Fax: 440-368-3569
- E-mail: info@spartanwatertreatment.com
Phenol and Benzoic Acid
Phenol and benzoic acid can be removed with ozone or advanced oxidation processes. The information on this page was taken from the following paper:
OXIDATION OF AROMATIC COMPOUNDS WITH UV RADIATION/OZONE/HYDROGEN PEROXIDE, A. MOKRINI, D. OUSSI, S. ESPLUGAS, Departamento de Ingenieria Quimica. Universidad de Barcelona, C/ Marti i Franquès, 1. 08028 Barcelona.
Key results of the study are the following:
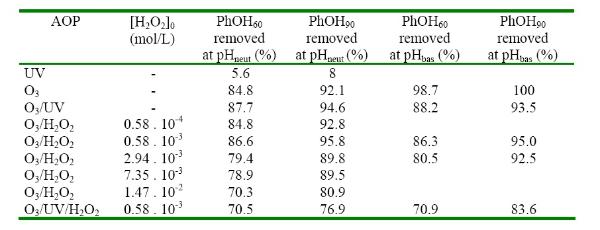
PhOH 60 and 90 are percentages of phenol removed after 60 and 90 min reaction time for each experiment. pH neut and pH bas are percentage remova rates at neutral pH (6.8-7.2) and basic pH (9.3-9.5)
Experiments at acidic pH
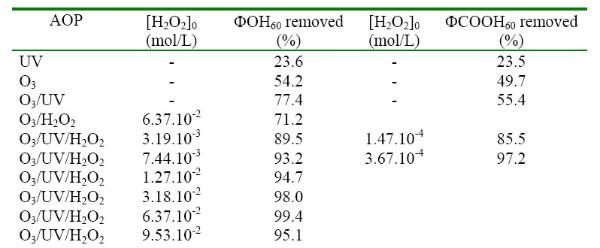
ΦOH60: pourcentage of phenol removed after 60 min reaction time.
ΦCOOH60: percentage of benzoic acid removed after 60 min reaction time.
Results show that phenol and benzoic acid can be significantly removed by ozone and advanced oxidation. pH is a significant factor in these reactions. Under acidic conditions, molecular ozone is the predominant reactant. ozone is a slower and more selective reactant. At elevated pH or in combination with peroxide and UV, the hydroxyl radical becomes the dominant reactant. These are advanced oxidation processes. The hydroxyl radical is a faster and less selective reactant.