There are many ways to disinfect and treat water, and ozone application is one of them. Here, we will discuss how ozone disinfects water along with its wide use in various industrial applications. Additionally, you will find technical information on how ozone disinfection compares to other commonly used disinfectants.

Ozone functions as both an oxidant and disinfectant in the treatment of drinking (potable) water and wastewater. This is similar to chlorine. Chlorine and ozone, however, operate by different mechanisms when disinfecting water. As a result, ozone and chlorine can act synergistically.
Ozone’s germicidal properties are associated with its high oxidation potential. Disinfection by ozone is a direct result of bacterial cell wall disintegration, also known as lysis. This mechanism is different than that by chlorine. Although the exact chemical action of chlorine is not clear, it is believed that the chlorine residual in aqueous solution diffuses through the cell wall of the microorganisms and attacks the enzyme group which results in the destruction of the microorganism.
The graphic below illustrates the microorganism destruction process:






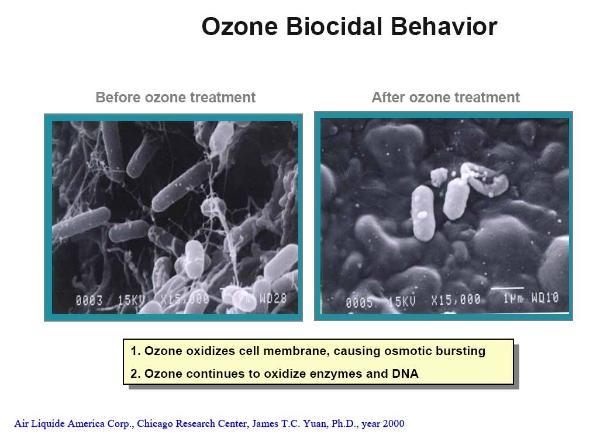
The photomicrograph shows actual bacteria before and after ozonation and demonstrates the biocidal behavior of ozone:
To demonstrate the disinfection power of ozone and compare it with other oxidizing agents, J.C. Morris developed the lethality coefficient:
Lethality Coefficient = 4.6/(Ct99) where:
C = residual concentration in mg/L
t99 = time in minutes for 99 percent microorganism destruction (2-log destruction)
Morris, J.C., “Aspects of the Quantitative Assessment of Germicidal Efficiency,” in Chap. 1, J.D. Johnson (ED.), Disinfection, Water and Wastewater, Ann Arbor Science, Ann Arbor, MI 1975.
The table below lists parameters for disinfection by ozone for different organisms:
| Parameters for Disinfection by Ozone (pH 7; 10-15 degrees C): | ||
|---|---|---|
| Organism | Lethality Coefficient (a) | C 99:10 (b) |
| Escherichia | 500 | 0.001 |
| Streptococcus faecalis | 300 | 0.0015 |
| Poliovirus | 50 | 0.01 |
| Endamoeba histolytica | 5 | 0.1 |
| Bacillus megatherium | 15 | 0.03 |
| Mycobacterium tuberculosam | 100 | 0.005 |
a – Lethality Coefficient = 4.6/(Ct99)
b – C 99:10 = concentration in mg/liter for 99 percent destruction or inactivation in 10 minutes
(Morris considered these values to be valid within a factor of two)
Learn more about specific microorganisms inactivated by ozone and fish pathogens inactivated by ozone.
Comparison of the values in this table with similar values obtained for chlorine is shown in the table below. These values tabulated by Morris illustrate that ozone is a more powerful germicide against all classes of organisms listed by factors of 10 to 100. The table shows values of the lethality coefficient:
| Lethality Coefficient at 5 degrees C [(mg/liter)-1(min.)-1] | ||||
|---|---|---|---|---|
| Agent | Enteric Bacteria | Amoebic cysts | Viruses | Spores |
| Ozone | 500 | 0.5 | <5 | 2 |
| HOCl as Chlorine | 20 | 0.05 | >1 | 0.05 |
| OCl- as Chlorine | 0.2 | 0.0005 | <0.02 | <0.0005 |
| NH2Cl as Chlorine | 0.1 | 0.02 | 0.005 | 0.001 |
Another way of looking at disinfection is to employ the CT concept. This associates a concentration of ozone multiplied using the CT concept.
Spartan Environmental Technologies supplies industrial ozone generators for a number of applications including: